Avoid Launch Delays By Planning For An FDA-Required REMS Risk
5 (262) · € 21.00 · En Stock
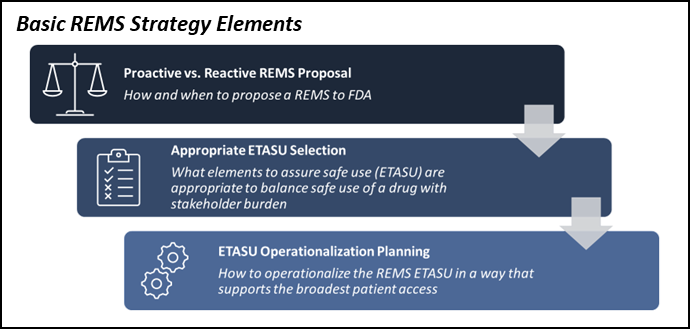
lt;p>Picture this: The FDA accepts a manufacturer's NDA, and the manufacturer plans for its impending launch. But shortly before the anticipated approval, the FDA notifies the manufacturer that a Risk Evaluation and Mitigation Strategy (REMS) program is required to market the product. Now what?</p>
From Our Perspective, A Two-Part Series: Risk Evaluation and Mitigation Strategies (REMS) Program

Improving Risk Evaluation and Mitigation Strategy - Cognizant

A Look Back at Risk Evaluation and Mitigation Strategies at the Food and Drug Administration in 2020: Year in Review - Food and Drug Law Institute (FDLI)
PDF) Risk Evaluation and Mitigation Strategies (REMSs): Are They Improving Drug Safety? A Critical Review of REMSs Requiring Elements to Assure Safe Use (ETASU)

White Paper, Missed Opportunities When Developing a REMS Program

Drug Safety and the Cost of Monitoring: The Role of REMS in Risk Management - Mark Slomiany, Rema Bitar, Sarah Kruse, Sarah Jeffers, Kenneth Berkowitz, Mahmud Hassan, 2015

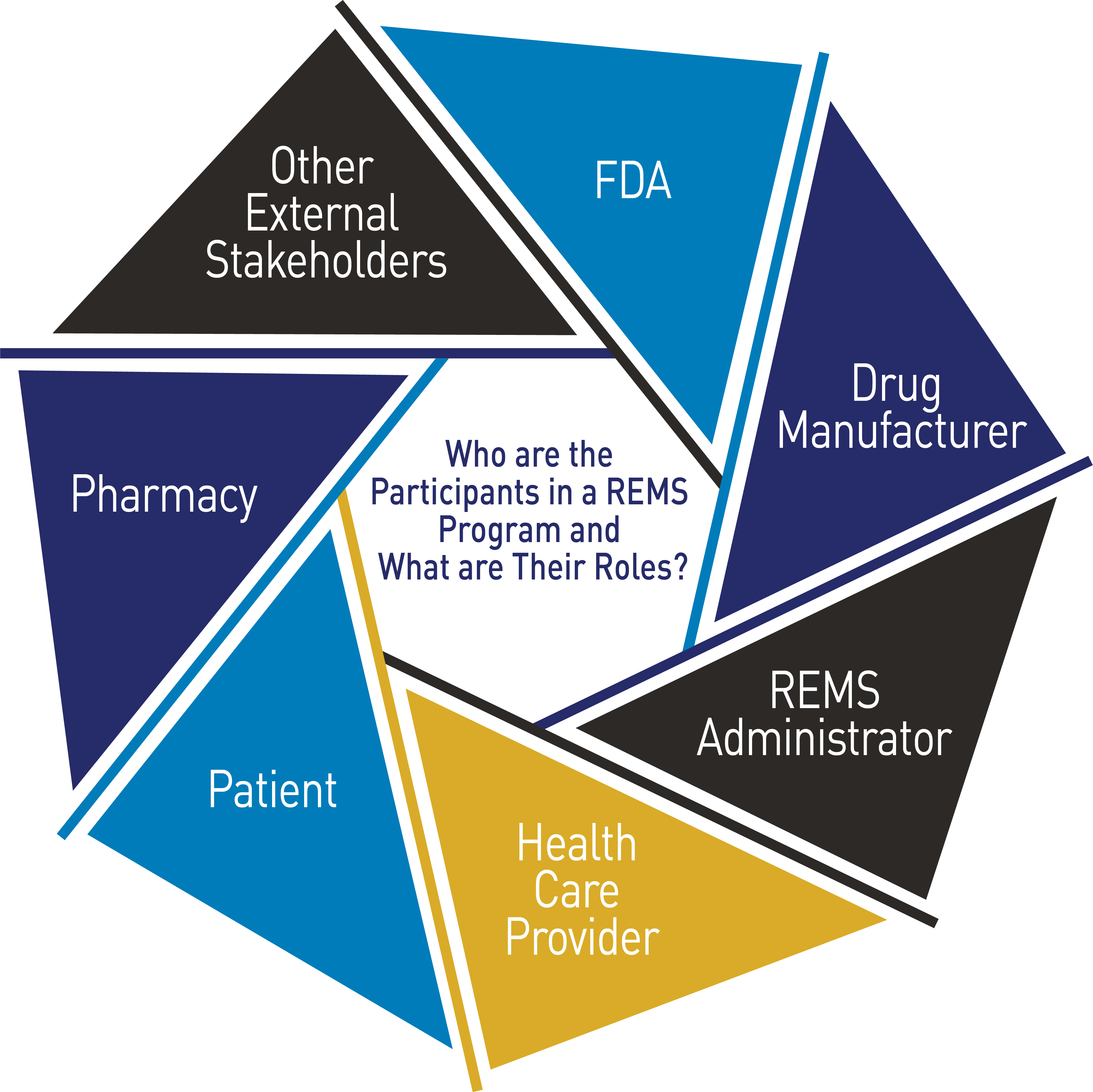
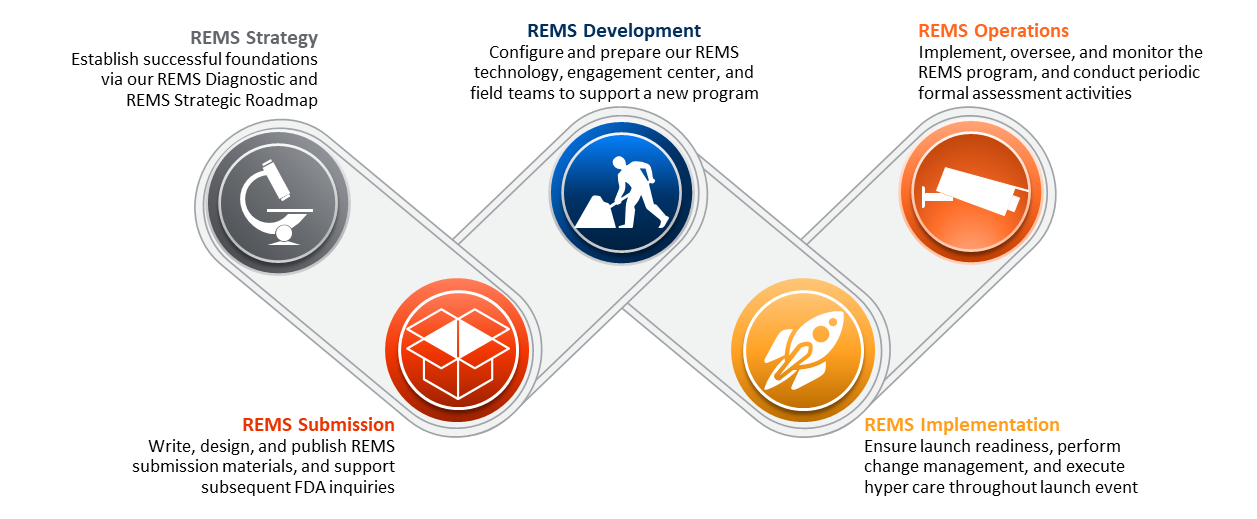
FDA's Risk Evaluation and Mitigation Strategies (REMS) Program: A Two-Part Series

Jazz Pharmaceuticals plc 2021 Directors' Report and Financial Statements

REMS Vendor Disruptions Prompt Greater US FDA Scrutiny :: Pink Sheet
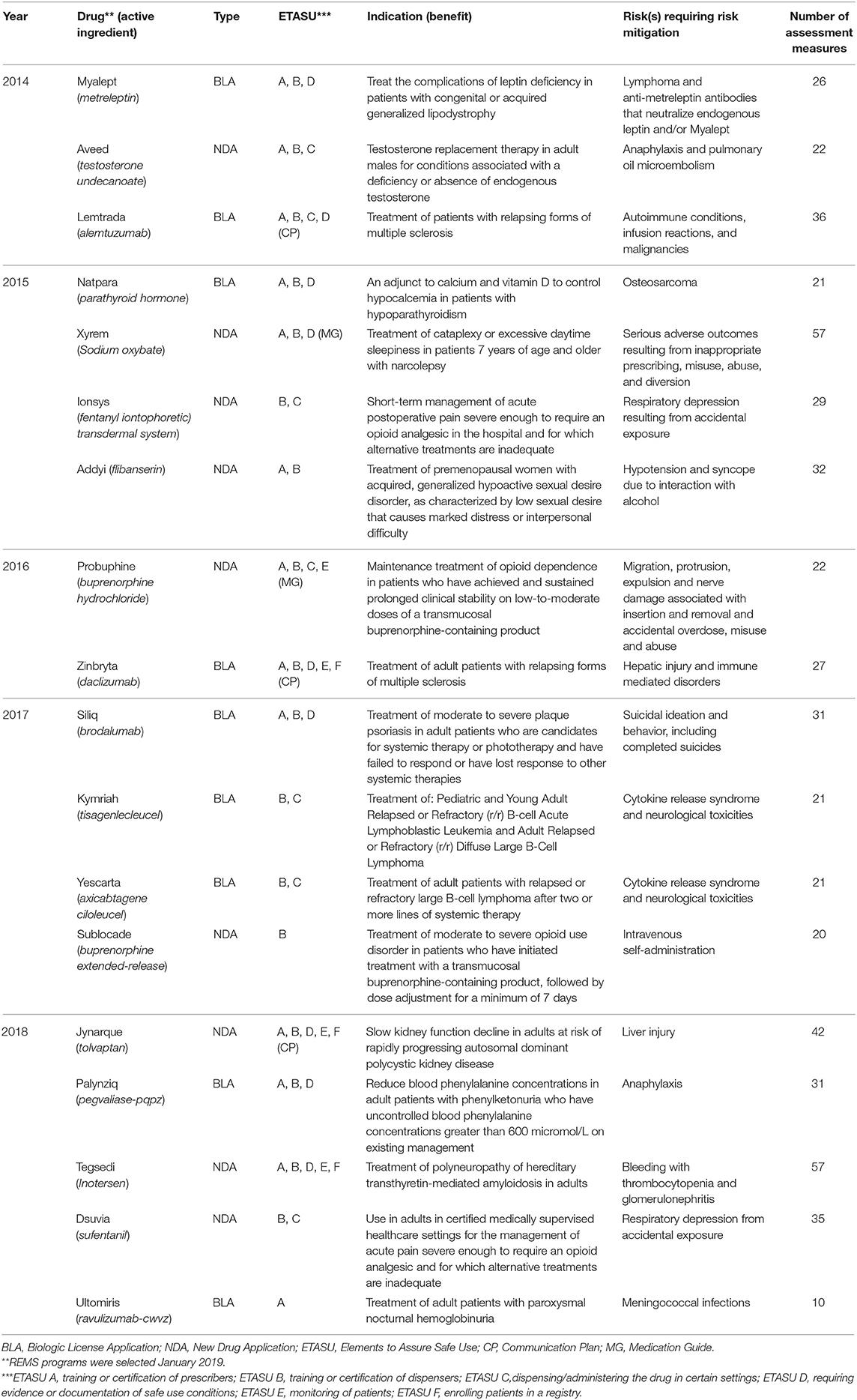
Frontiers Adaptation for Regulatory Application: A Content Analysis of FDA Risk Evaluation and Mitigation Strategies Assessment Plans (2014–2018) Using RE-AIM
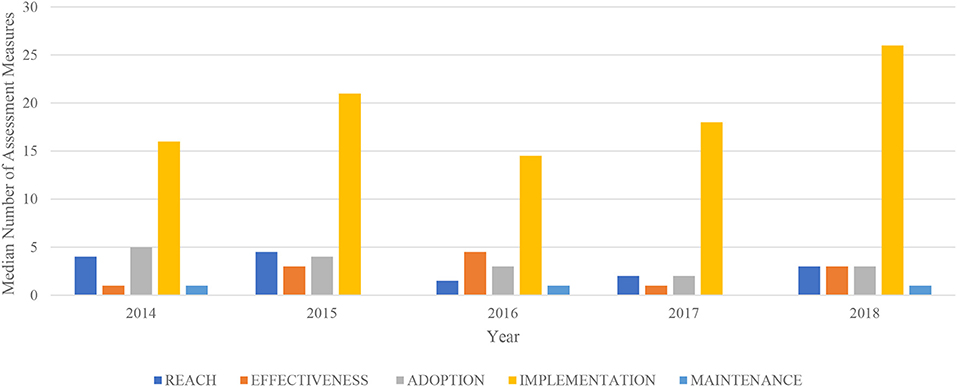
Frontiers Adaptation for Regulatory Application: A Content Analysis of FDA Risk Evaluation and Mitigation Strategies Assessment Plans (2014–2018) Using RE-AIM

Table 3 from Risk Evaluation and Mitigation Strategies (REMS)

Risk Evaluation and Mitigation Strategies (REMS)

2 Incorporating Benefit and Risk Assessment and BenefitRisk Management into Food and Drug Administration Decision-Making, Ethical and Scientific Issues in Studying the Safety of Approved Drugs